Rising Trends: Growth in Investment Arbitration in the Mining Industry in the last five years
Ljiljana Madzarevic2024-01-22T16:15:50+01:00
Between 1996 and 31 December 2023 61 arbitration cases involving mining concessions were registered before ICSID.
Of these it is notable that 36 cases have been registered within the last five years, specifically in the period from 1 January 2019 to 31 December 2023 – more than 50% percent of all arbitration proceedings registered before ICSID, indicating a significant shift in the landscape of investment arbitration in the mining sector.
32 of the 61 cases registered before ICSID have now been concluded with the following outcomes:
- 12 were discontinued under ICSID rules;
- 5 had judgments rendered in favor of the investor, with damages awarded amounting to USD tens of millions;
- 8 were decided in favour of the state;
- 4 were resolved through settlement;
- 3 have undetermined outcomes.
Among the documents invoked, investors predominantly relied on Bilateral Investment Treaties (BITs), followed by contracts and free trade agreements. A detailed overview of the documentation cited can be found in the chart below:
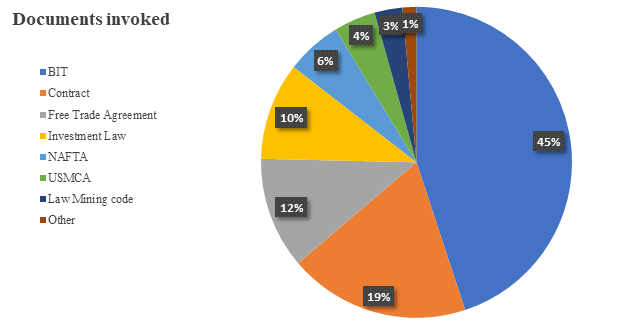
BITs were predominantly invoked by investors from the UK (including Northern Ireland), followed by those from the Netherlands, the US, Switzerland and Canada. A detailed overview of the nationalities of investors initiating these arbitration proceedings is shown in the chart below:
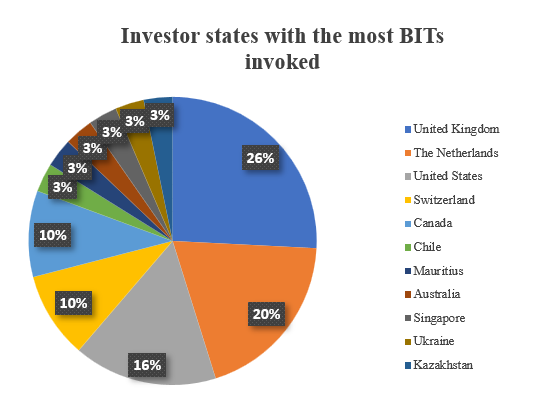
Looking at the countries that faced the highest number of filed claims, Colombia takes first place with 15% of all ICSID arbitration proceedings concerning mining concessions, followed by the Democratic Republic of Congo standing at 13% with Mexico and Venezuela at 7%.
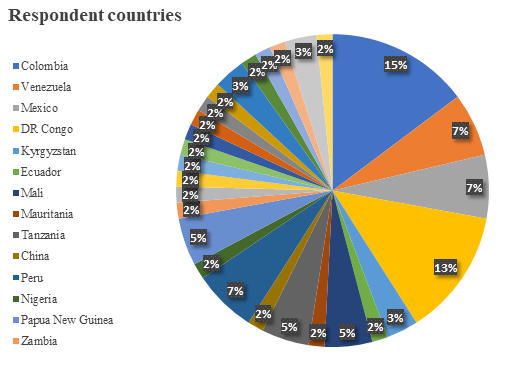
These statistics make it clear that the number of arbitration proceedings initiated in the context of mining concessions have been rapidly increasing over the past five years. The fact that the mining industry is developing rapidly in response to […]










